
Uncategorised
 Calvin Bridges is, with Alfred Sturtevant, one of my heroes of Drosophila genetics. Among his achievements were the demonstration and confirmation of the chromsome theory of inheritance, and the establishment of the polytene chromosome maps (for more about polytene chromosomes, see this article). Bridges was one of the early members of the Morgan fly lab, and stayed there for his entire (though unfortunately short) career. Kohler, in his excellent history of Drosophila genetics, characterises Bridges as the "blue collar" member of the lab, the worker who would invest huge energy in the technical development of Drosophila genetics.
Calvin Bridges is, with Alfred Sturtevant, one of my heroes of Drosophila genetics. Among his achievements were the demonstration and confirmation of the chromsome theory of inheritance, and the establishment of the polytene chromosome maps (for more about polytene chromosomes, see this article). Bridges was one of the early members of the Morgan fly lab, and stayed there for his entire (though unfortunately short) career. Kohler, in his excellent history of Drosophila genetics, characterises Bridges as the "blue collar" member of the lab, the worker who would invest huge energy in the technical development of Drosophila genetics.
It was Bridges who:
- devised a standard fly medium of cornmeal-agar-molasses (originally the flies were maintained ona banana mush)
- devised Drosophila incubators
- established the Drosophila Information Service (DIS)
Bridges' PhD thesis was on "Non-disjunction as proof of the chromosome theory of heredity", and was published as the first article in the first issue of the journal Genetics. He is best remembered for his polytene chromosome maps. Polytene chromosomes are rather peculiar structures, first recognised by Balbiani in the 19th century, which at first sight don't really look like chromosomes. They are formed by the repeated replication of chromosomes (endoreduplication), where all the copies stay synapes in precise alignment. In the case of the third instar salivary gland cells, the level of endoreduplication is of the order of 1000x. The resulting structures display a reproducible banding pattern: this forms the basis of Bridges' inspired chromosome maps. In fact Bridges wasn't the first to use polytene chromosomes for genetic mapping: Theophilus Painter mapped chromosome inversions in a paper in Science in 1933. However, it wasn't until Bridges devised his mapping system that Drosophila resarchers the world over could unambiguously refer to specific loci on the polytene chromosomes. These maps were published in 1935, and are still key in rsearch today.
Bridges had a somewhat unconventional lifestyle (some would say "flamboyant"). He left his wife and family to pursue "free love", in which his main strategy was apparently to do a lot of propositioning. This of course led him into some complicated scrapes, some of which are described in Kohler's "Lords of the Fly": Kohler says "he was famous for his flamboyant lifestyle; he left his wife and children in the mid-20s (but continued to support them), got a batchelor pad and a vasectomy; propositioned every woman he met, indiscriminately... his extraordinary good looks and innocence were irresistible" (Kohler 1994 p113).
In the 1925 catalogue of 365 Drosophila mutants, Bridges was responible for the discovery of about two thirds. Indeed Hermann Mullert remarked that Bridgeswas so prolific a mutant discoverer because he set up and scored as many crosses as all the other lab members combined!
Below: Calvin Bridges in the Carnegie Fly Lab. Note the "totem pole", on which the genetic maps were assembled, behind him.

Bridges' paper on chromosome theory from 1916, published in Genetics. This appears to be freely available.
Bridges' polytene chromosome map: Bridges (1935) Salivary Chromosome Maps: With a Key to the Banding of the Chromosomes of Drosophila melanogaster. J. Hered., February 1935; 26: 60 - 64. Link to PDF It appears that a subscription is required to view this classic paper. Shame on the journal. The paper is 73 years old, and it's 50 years since Bridges died. These old and classic papers in journals should not be restricted in this way.
 Bad Blood: The Secret Life of the Tour De France
Bad Blood: The Secret Life of the Tour De France
Jeremy Whittle
Pub Yellow Jersey Press, 2008
Jeremy Whittle is a widely published cycling journalist, and as the blurb on the back cover says, this is an account of his transition from hard core fan of cycling sport (as epitomised by the greatest sporting event, the Tour de France), to a position of strong scepticism. The author's relationship with professional cyclists has been on a personal level and there's a definite sense of disappointment at the level of duplicity at all levels of the sport, from the UCI's unwillingnness to deal with widespread doping, to the teams washing their hands of responsibility, to the riders who actually practise the doping.
There's a sense also of the PR machine that teams such as US Postal/Discovery set up up to protect the interests of their star riders such as Lance Armstrong. It seems pretty clear that Whittle believes that institutional doping is at the heart of some teams' success, and indeed the way that USP/Disco and Armstrong ruled the peleton (and the often rather petty vendettas against riders prepared to speak out against doping) can be intepreted as a support for the prevailing doping practices. The book is really quite touching as you read about Whittle's changing relationship with riders such as David Millar, and former riders such as Paul Kimmage.
An excellent book for the follower of professional cycling.

Back when I was a carefree postdoc, one of the projects I worked on was the assembly of a molecular physical map of the Drosophila melanogaster genome. Of course, Drosophila researchers had for years been using a physical map, the polytene chromosome map, and indeed we used this as the framework on which we assembled our molecular map using cosmid clones. These papers take the genome sequences of 11 Drosophila species (plus the sequence of Drosophila melanogaster, determined back in 2000), fit them to the polytene chromosome maps, and examine chromosome rearrangments seen in inter-species comparisons. It seems to me there isn't anything hugely sexy in this work, but there is a huge amount of work that sets the evolutionary relationships between these Drosopholids in context. It's also an opportunity to expound on chromosomes in Drosophila!
Polytene chromosomes are unusual structures - originally found by Balbiani in the 19th century in Chironomus, they are in fact highly endroreduplicated chromosomes. More than that, all the chromosome copies are in an interphase state and precisely aligned, resulting long rope-like structures with highly reproducible banding patterns (these bands are the basis on which polytene chromosome maps are devised).
Polytene chromosome s are found in a variety of tissues in the true flies (Diptera), and perhaps reach there greatest lab utility in Drosophila melanogaster. The picture on the left shows a complete set of D. melanogaster polytene chromosomes - note that each centromere and the pericentromeric heterochromatin (which is not amplified) are clustered to form the chromocentre, from which each of the chromosome arms radiate (though the animal is diploid, both homologues are tightly paired - so for example both 2L arms form one structure. This summarised in the diagram below.
s are found in a variety of tissues in the true flies (Diptera), and perhaps reach there greatest lab utility in Drosophila melanogaster. The picture on the left shows a complete set of D. melanogaster polytene chromosomes - note that each centromere and the pericentromeric heterochromatin (which is not amplified) are clustered to form the chromocentre, from which each of the chromosome arms radiate (though the animal is diploid, both homologues are tightly paired - so for example both 2L arms form one structure. This summarised in the diagram below.
 Panel (a) shows the female mitotic karyotype, with the arms identifiable by labels and colours. The male karyotype has a single X chromosome paired with a heterochromatic Y chromosome. Panel (b) sows how these arms relate to the polytene chromosomes (no to scale). Notice that despite the animal being diploid, each pair of chromosome arms is represented by a single arm in the polytene configuration. In the mal, the Y chromosome remains part of the chromocentre, along with the centromeres and pericentromeric heterochromatin.
Panel (a) shows the female mitotic karyotype, with the arms identifiable by labels and colours. The male karyotype has a single X chromosome paired with a heterochromatic Y chromosome. Panel (b) sows how these arms relate to the polytene chromosomes (no to scale). Notice that despite the animal being diploid, each pair of chromosome arms is represented by a single arm in the polytene configuration. In the mal, the Y chromosome remains part of the chromocentre, along with the centromeres and pericentromeric heterochromatin.
Thomas Painter originally used polytene chromosomes to map genetic variants, but it was Calvin Bridges who devised the first usable map: each of the five chromosome arms were divided into 20 divisions, numbered 1 to 102 (the tiny fourth chromosome has only two divisions), and each division was subdivided into 6 subdivisions, indicated by the letters A-B. In later revisions, Bridges numbered each individual band of each subdivisions. Considering this work was conducted by light microscopy, it is remarkable that the map can still be used today, and indeed has been supported by electron microscopy. It represents a real tour de force of cytogenetic mapping, and is the prototype map for a variety of insect species.
The image above shows a couple of divisions (i.e. about 2%) of Bridges' polytene map. The polytene map permitted detailed mapping of chromosome rearrangements such as deficiencies, inversions, etc; and from the early days of moleclar genetics direct localisation of cloned genes by in situ hybridisation. And, of course, they played their part in evolutionary studies, particularly in other Drosophila species.
The D. melanogaster polytene map is in many ways the "gold standard" map: the most highly used, and indeed the most detailed. For other Drosophila species, maps are not necessarily constructed in the same way in terms of nomenclature.
Muller proposed that the chromosomes of Drosophila species correspond to a set of "elements" - essentially representing syntenic blocks. Muller named these elements A-F: in D. melanogaster element A corresponds to the X chromosome, element B to the left arm of chromosome 2, B to the right arm of chromosome 2 and so forth.
The diagram below (click for a larger version) illustrates the correspondence between Muller's elements and the karyotypes of the species featured in these papers.
A large scale effort to sequence the genomes of several Drosophila species now means that in addition to D. melanogaster, the genomes of a further 11 species have been determined. All species in the phylogeny above have been sequenced.
So, on to the first paper, Polytene Chromosomal Maps of 11 Drosophila Species: The Order of Genomic Scaffolds Inferred From Genetic and Physical Maps. This paper aligns the species scaffolds against the polytene chromosome maps (and in some cases updating the polytene chromosome maps), with the aim of contributing to investigating five questions:
What is the molecular basis of differences between these species?; what is the mechanistic basis of distinct sex chromosomes?; How do new inversions originate?; What is the basis of gene arrangement polymorphisms within a species?; and finally, why in some species do gene arrangement polymorphisms occur only on some of the chromosome arms?
< p>By using both syntenic analysis and physical mapping, the sequence scaffold blocks for each of the 11 species studied have been aligned to their respective polytene chromosome maps. This represents a lot of work - there are many supplementary documents (which I confess I've not read). The genome sizes of these species vary widely: from 236.6 Mb for D. willistoni to 137.8 Mb in D. simulans. These differences are principally due to differing amounts of heterochromatic or other unassigned DNA located around centromeres.The second paper, Chromosomal Rearrangement Inferred From Comparisons of 12 Drosophila Genomes, describes the patterns ofchromosome rearrangements in a phylogenetic context. They also address structural genome features associated with chromosome inversion breakpoints, in particular in those species such as D. pseudoobscura which have well characterised intraspecific inversion polymorphisms.
The figure below is a schematic showing how chromosome inversions and other rearrangents map out on Muller's elements. You can click on the image for a bigger version (may need subscription).
Each element is composed of a set of hues, and inverted segments are linked by lines. In fact single genes are represented by single lines, andare coloured in blocks of a hundered or so genes in D. grimshawi. It's a neat graphic that cleverly shows that the vast majority of rearrangementshave occurred within single elements: a notable exception is the translocation between D and A in D. psuedoobscura (and D. persimilis).
One of the things I was interested to glean from these studies was the nature of inversion breakpoints. Some years ago I worked on the malaria mosquito Anopheles gambiae - this species isactually a species complex of 6 species pretty well indistinguishable on morphological criteria, but which were clearly reproductively isolated (at least in the wild) on the basis of the distribution of chromosome inversions between the species. Many of these inversions share breakpoints, at least at the level of polytene cytology. One inference was that these breakpoints were seen multiple times because there was a hotspot, possibly due to some feature in the DNA - possibly a transposable element or a segment of heterochromatin. The same situation is, I think, seen in the polymorphic inversions of D. pseudoobscura, which have been studied in considerable detail, as they seem to vary in frequency depending on environmantal factors such as altitude, temperature and season. What do Bhutkar et al say that is relevant to this?
Of course, as with the rest of the paper, deep understanding (at least for me) is made difficult by the fact that much of the terminology is from a mathematical or computing biology source. However, as far as I understand it, they've analysed patterns of breakpoint reuse, as judged by mapping them to boundaries between syntenic blocks. I presume that direct comparison between species' sequences is difficult due to sequence divergence. It follows then that apparent beakpoint reuse may only reflect the distance between syntenic blocks. The prospect that is also raised is that some regions may lack breakpoints because they would split coordinately expressed blocks of genes (previous workers had identified long domains of genes with similar or identical expression patterns, and the inference was that they were co-ordinately expressed.
Bhutkar et al conclude on the basis of both synteny analysis and computational modelling that breakpoint reuse does occur. This in turn implies that some feature of the chromosome is responsible. One hypothesis that was attractive was that transposable elements would turn out to be responsible for the formation of inversions- however, many studies in a variety of systems havefailed to support this, though in many cases, reptitive sequences do localise to inversion breakpoints. The evidence presented here does not support elevation of repeat sequences at breakpoints, though the authors do note that the present situation need not reflect the situation at the time an inversion arose.
A second hypothesis for breakpoint formation was proposed by Novitski (1946). Novitski proposed that synapsis of inverted and standard arrangement chromosomes would lead to elevated frequencies of double strand breaks in the vicinity of breakpoints - consistent with observed patterns of breakpoints within and between species. the sequence of events would use progressively more distal breakpoints. Unfortnately the data to date are not clear enough on the sequential nature of inversions to provide support (or not) for Novitski's hypothesis.
I don't know where the studies of these genome sequences are going, but it would be of interest if some level of analyses could be applied to D. pseudoobscura and its intraspecific inversion polymorphisms. Richards et al (2005), in the publication describing the D. pseudoobscura genome sequence look at inversions in this species and frequently find repetitive DNA at inversion breakpoints. In one case, the intraspecific polymorphic Arrowhead inversion (in Muller element C), and molecular evidence implicates short inverted repeat sequences in the formation of the inversion.
----------------------------------------------------------------------------------------------
 I was intrigued by a brief news piece in the latest issue of Science to fall onto my desk (the 22nd August issue). This concerns the recently published genome sequence of Trichoplax adhaerens, a peculiar animal in a phylum I'd never heard of. That in itself was interesting, particularly as placozoans have a really odd body plan that involves a mere four cell types. Wikipedia has a nice description of Placozoa, from which the image below comes.
I was intrigued by a brief news piece in the latest issue of Science to fall onto my desk (the 22nd August issue). This concerns the recently published genome sequence of Trichoplax adhaerens, a peculiar animal in a phylum I'd never heard of. That in itself was interesting, particularly as placozoans have a really odd body plan that involves a mere four cell types. Wikipedia has a nice description of Placozoa, from which the image below comes.
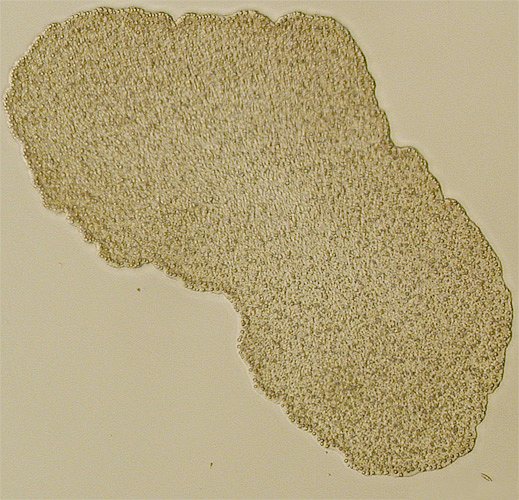
On browsing the web a bit further, I found this movie (Quicktime format) of a placozoan moving. I presume this would be Trichoplax adhaerens, as this is the only known species in the phylum - a second described species, T. reptans, was apparently described at the end of the 19th century but hasn't ben seen again and it's existence is doubtful.
Trichoplax appears to have no sensory cells, muscle cells, or a nervous system - it glides over the substrate using cilited cells on its underside, secreting enzymes to digest food - food is digested externally and via phagocytosis.
The full citation for the genome paper is Srivastava et al (2008) The Trichoplax genome and the nature of placozoans. Nature 454;955 (doi:10.1038/nature07191). Full Text; pdf (The paper is released under the creative commons licence, so should be available)
The general picture about the Trichoplax genome is: 98 megabases, distributed over six chromosomes, and containing 11,514 protein coding genes (only a couple of thousand fewer than my favourite system, Drosophila). The sequence has answered quite a few questions about where the phylum Placozoa fits into the animal kingdom: it appears they branched after sponges, but before Cnidaria (the jellyfishes, corals and sea anemones). There may well be more complexity within the apparently simple tissue structures of these organisms. Trichoplax has a wider variety of genes than one might expect for such a simple body plan: this might reflect the origins of genes which are used inthe development of a wide variety of cell types in more complex organisms. Alternatively, Placozoa may merely be a phase in an otherwise undiscovered life cycle.
Above: the deduced phylogenetic position of Placozoa (from Srivastava et al (2008).
Mansi Srivastava, Emina Begovic, Jarrod Chapman, Nicholas H. Putnam, Uffe Hellsten, Takeshi Kawashima, Alan Kuo, Therese Mitros, Asaf Salamov, Meredith L. Carpenter, Ana Y. Signorovitch, Maria A. Moreno, Kai Kamm, Jane Grimwood, Jeremy Schmutz, Harris Shapiro, Igor V. Grigoriev, Leo W. Buss, Bernd Schierwater, Stephen L. Dellaporta, Daniel S. Rokhsar (2008). The Trichoplax genome and the nature of placozoans Nature, 454 (7207), 955-960 DOI: 10.1038/nature07191

